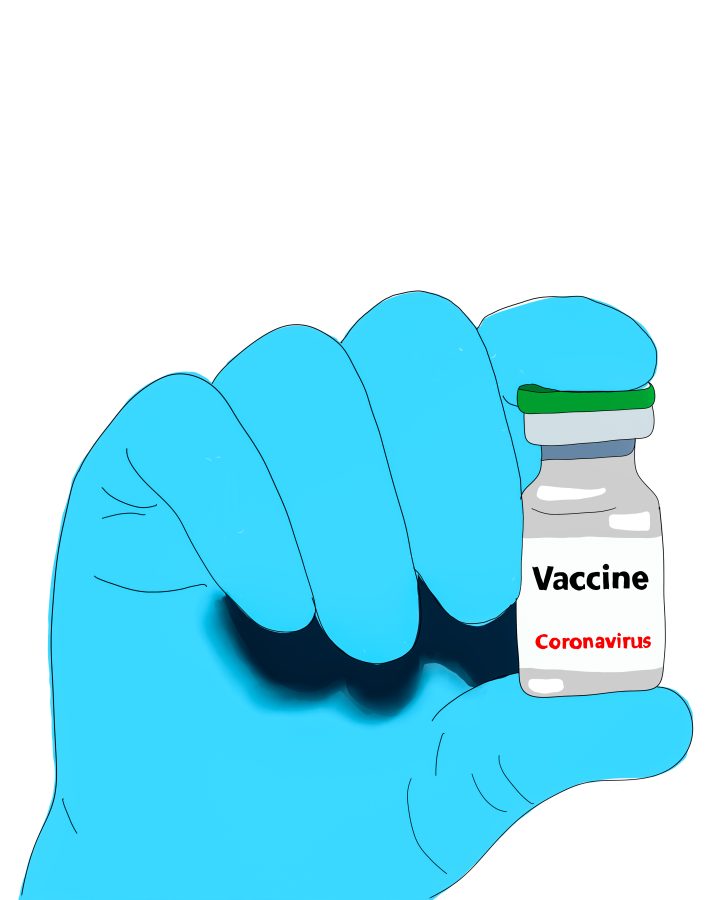
After a brief pause on the usage of the Johnson & Johnson COVID-19 vaccine, the U.S. Food and Drug Administration and the U.S. Center for Disease Control and Prevention have determined that use of the vaccine should continue.
The pause was announced on April 13. The halt on the vaccine occurred due to reports of the development of a rare and severe blood clot in six individuals six to 13 days after they received the Johnson & Johnson shot. All of the cases reported afflicted women between the ages of 18 and 48.
At that point, over 6.8 million doses of the Johnson & Johnson vaccine had already been administered in the U.S. However, the CDC and the FDA wrote in their joint April 13 statement that despite the perceived rarity of the blood clots, “COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of health problems following COVID-19 vaccination very seriously.”
On April 23, the Advisory Committee for Immunization Practices voted 10 to four to resume administration of the vaccine for people aged 18 and above. Following the vote, the FDA and CDC released another joint statement which detailed their “thorough safety review” of the vaccine and updates regarding the adverse events reported, and stated that safety monitoring of the vaccine will continue.
Including the original six cases of the severe blood clots, 15 total cases have been reported to the Vaccine Adverse Event Reporting System. The cases still only occurred in women, however the age range extended to 18-59. The time period for onset of symptoms also extended from 13 days at the latest to 15 days.
“We’ve lifted the pause based on the FDA and CDC’s review of all available data and in consultation with medical experts and based on recommendations from the CDC’s Advisory Committee on Immunization Practices,” said Acting FDA Commissioner Janet Woodcock, M.D. “We have concluded that the known and potential benefits of the Janssen [Johnson & Johnson] COVID-19 Vaccine outweigh its known and potential risks in individuals 18 years of age and older.”
Dr. Rochelle Walensky, Director of the CDC, also expressed her support of the ACIP’s recommendation to lift the pause of the Johnson & Johnson vaccine.
“For every one million doses of this vaccine, the J&J vaccine could prevent over 650 hospitalizations and 12 deaths among women aged 18 to 49, and this vaccine could prevent over 4,700 hospitalizations and nearly 600 deaths among women over 50,” said Dr. Walensky.
The blood clot disorder has been identified as thrombosis with thrombocytopenia syndrome. According to health officials, seven of the women who developed the syndrome were obese, two of the women were using oral contraceptives, and two suffered from high blood pressure. Three of the afflicted women died.
Along with lifting the pause, the CDC has added a warning label for extremely rare blood clots. Additionally, the CDC has emphasized the importance of telling women the risks associated with the vaccine so they could make an informed decision about whether or not they want to receive the vaccine.
As of April 25, 31 states had either resumed or announced plans to resume usage of the Johnson & Johnson vaccine. Administration of the Johnson & Johnson vaccine resumed in Massachusetts on Friday, April 23, very shortly after the CDC and FDA announced their joint statement.